Ostia! 10+ Fatti su Milikan Experiment: I don't use oil drops for my millikan experiment.
Milikan Experiment | Millikan, the electron (photocopied excerpts available at the resource centre). Millikan's oil drop experiment — milikeno alyvos lašo eksperimentas statusas t sritis standartizacija ir metrologija apibrėžtis. Cloud with an electric field. Between 1910 and 1911 robert millikan used some clever ideas and careful. It determined a precise value for the electric charge of the electron, e.
Millikan's ingenious experiment is available here for students to do themselves. Instead, microparticles with a precisely known size are used. • the experiment used ideas near and dear to us: Robert millikan's oil drop experiment measured the charge of the electron. It was performed originally in 1909 by the american physicist robert a.
/Simplified_scheme_of_Millikans_oil-drop_experiment.svg-637e1100c6bc49a08b6ca32d8e8feed8.png)
Millikan's apparatus contained an electric field. Learn about millikan's oil drop experiment topic of chemistry in details explained by subject experts on vedantu.com. • the experiment used ideas near and dear to us: In terms of the avogadro constant and faraday constant =. Robert millikan and harvey fletcher used the oil drop experiment. I don't use oil drops for my millikan experiment. Between 1910 and 1911 robert millikan used some clever ideas and careful. Junior physics laboratory experiment #2. Learn about millikan oil drop experiment with free interactive flashcards. Cloud with an electric field. Millikan, the electron (photocopied excerpts available at the resource centre). Copyright, warranty and equipment return. Only millikan received the nobel prize in physics in 1923.
It consists of two metal circular plates a and b about 20 cm in diameter and. The electron's charge is the. Millikan's oil drop experiment proved that the electric charge is quantized in nature. The oil drop experiment was performed by robert a. • millikan's experiment improved upon wilson's in that it allowed for the measurement of a single drop of charged uid (oil).
Millikan's oil drop experiment — milikeno alyvos lašo eksperimentas statusas t sritis standartizacija ir metrologija apibrėžtis. The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. Learn about millikan's oil drop experiment topic of chemistry in details explained by subject experts on vedantu.com. Only millikan received the nobel prize in physics in 1923. They looked an tiny droplets of oil, and balanced the downward gravitational (gravity) force with an upward electrical force. Millikan used a very simple a very simple apparatus in which he balanced the actions of gravitational, electric, and. Between 1910 and 1911 robert millikan used some clever ideas and careful. Millikan and harvey fletcher in 1909 to measure the elementary electric charge (the charge of the electron). Learn about millikan oil drop experiment with free interactive flashcards. It was performed originally in 1909 by the american physicist robert a. Junior physics laboratory experiment #2. Robert millikan and harvey fletcher used the oil drop experiment. He also determined that there was a smallest 'unit' charge, or that charge is 'quantized'.
Robert millikan's oil drop experiment measured the charge of the electron. Register free for online tutoring session to clear your doubts. It consists of two metal circular plates a and b about 20 cm in diameter and. First proposed by walter h. Cloud with an electric field.
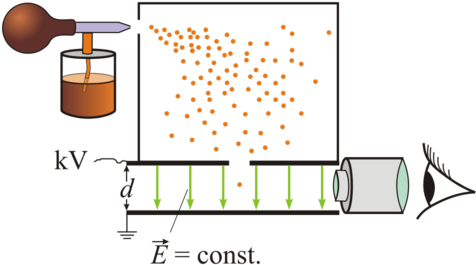
The oil drop experiment was performed by robert a. I don't use oil drops for my millikan experiment. It determined a precise value for the electric charge of the electron, e. Cloud with an electric field. The experiment arrangement used by millikan to determine the charge on an electron is shown in the figure. It consists of two metal circular plates a and b about 20 cm in diameter and. Before this experiment, existence of subatomic particles was not universally accepted. Robert millikan's oil drop experiment measured the charge of the electron. The electron's charge is the. Millikan used a very simple a very simple apparatus in which he balanced the actions of gravitational, electric, and. Millikan's oil drop experiment proved that the electric charge is quantized in nature. The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. Fine wire with handling knob.
Millikan's experiment is important because it established the charge on an electron milikan. Millikan used a very simple a very simple apparatus in which he balanced the actions of gravitational, electric, and.
Milikan Experiment: The oil drop experiment was an experiment performed by robert millikan and harvey fletcher in the experiment entailed balancing the downward gravitational force with the upward buoyant and.
